You may have covered in your elementary school chemistry course that some elements in nature may form various allotropes, simply it refers to different structural forms of the same element.

Carbon among all the 118 elements in the periodic table is a fundamental building block of many vital molecules that compose life on earth, for example the DNA the double helical molecule formed of different nucleotides (adenine, cytosine, thymine and gaunine), another molecules are carbohydrates, proteins and many of other important organic compounds.

Another interesting forms of pure carbon are the two allotropes diamond and graphite. Yes don’t be shocked the two forms that are in terms of economic value very different are in reality structured from the same element carbon. So what differentiate graphite from diamond? It is chemistry! The fascinating world of studying matter, it’s structure and the changes that happens to it.

Graphite as we know is a black and very soft material used to manufacture lead pencils. What explains this soft behavior is nothing other than the weak chemical bonds between the single layers of graphene composing the graphite, where each layer of graphite called “graphene” is a matrix of carbon atoms bonded to each other in a hexagonal pattern. On the other hand, diamond which is also made of pure carbon that are structured in 3D space in a complex compact pattern which explains why diamond is one of the hardest materials on earth and is used other than luxury jewels, in manufacturing of medical surgery equipment, also used in making drilling bits used to drill oil and gas wells and many other uses.. So interesting to know that it’s just a matter of structure which differentiate an expensive diamond from a cheap graphite.
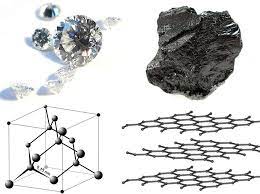
What determines how the same element can take different forms? It is just the physical properties such as temperature and pressure to which the element is exposed. Diamond is formed in extreme geological conditions in the crust of the earth when carbon is exposed to high pressures and high temperatures which forces carbon atoms to take its complex 3D structure, while graphite being is formed in less extreme medium.

Scientists in the lab had succeeded to grow synthetic diamonds from graphite in well engineered devices (HPHT) that can simulate the extreme conditions of pressure and temperature happening deep in the earth. There is a high probability that the diamond ring of your wife is from a synthetic origin! But for your benefit synthetic diamonds are not in the purity of natural diamonds, so it is easily detected by those working in jewelry.
Away from chemistry which reveals us some lessons and projections from matter, it is nice to know that hard working in extreme conditions towards noble goals is what differentaite a successful person from failure person who is still in his comfort zone.
Be a diamond in a world full of graphites!
Written by Ali Nasser
1 September 2022